2020 Volume 45 Issue 3 Pages 151-162
2020 Volume 45 Issue 3 Pages 151-162
Information on the safety of chemical substances in patients with various preexisting conditions remains limited. Acetaminophen was added to the basal diet at 0, 80, 253, 800, 2530, or 8000 ppm and administered to type 2 diabetes mellitus rats (GK/Jcl) and the control male rats (Wistar) for 13 weeks. Both strains treated with 8000 ppm acetaminophen (561.4 and 567.7 mg/kg body weight/day, GK/Jcl and Wistar rats, respectively) showed decreased levels of red blood cell counts, blood urea nitrogen, creatinine, and total bilirubin compared to those of non-treated rats. Treatment with 8000 ppm of acetaminophen reduced the blood glucose and hemoglobin A1c levels of GK/Jcl rats. An increase in the relative weights of the kidneys and liver, and a decrease in the weight of the salivary glands were observed in both GK/Jcl and Wistar rats treated with 8000 ppm acetaminophen relative to those of non-treated control rats. Microscopically, both strains treated with 2530 (174.3 and 164.2 mg/kg body weight/day, GK/Jcl and Wistar rats, respectively) or 8000 ppm acetaminophen showed hepatocellular hypertrophy and degenerative lesions in the salivary glands, whereas similar lesions were not observed in non-treated rats. In conclusion, the no-observed-adverse-effect-level of acetaminophen was 800 ppm in both diabetic and control rats.
Repeated-dose oral toxicity studies have been performed to determine the no-observed-adverse-effect-level (NOAEL) of chemicals, including pharmaceuticals and food ingredients, usually using healthy and genetically uniform animals. Various common health conditions such as type 2 diabetes mellitus (T2DM), obesity, hyperlipidemia, and hypertension (Younossi et al., 2016) directly or indirectly affect the metabolic and excretory functions of the liver, kidney, and other organs (Gray et al., 2014). In addition, cumulative epidemiological and experimental studies have shown that T2DM increases the risk of cancer and cancer-related mortality (Gallagher and LeRoith, 2015). Although T2DM is increasing worldwide due to lifestyle changes and increasingly aging societies, experimental studies examining the relationship between T2DM and the susceptibility to chemical toxicity are limited. T2DM is the most common metabolic disorder in humans, and it is often associated with renal dysfunction. Therefore, it is important to evaluate the toxicity of various chemicals using diabetic models.
GK/Jcl rat was established by selectively breeding Wistar rats with low glucose tolerance, and glucose levels were measured using the oral glucose tolerance test (Goto et al., 1976; Sugawara et al., 2004). These rats are not obese and have high blood glucose levels (200 mg/dL), low insulin secretion determined by testing glucose tolerance, and mild insulin resistance. As a result, they have been used as an animal model of insulin-resistant T2DM without obesity, which are common characteristics of T2DM in the Japanese population.
Acetaminophen (APAP, paracetamol) is one of the most commonly used drugs for the treatment of pain and fever in both humans and animals, and is mainly metabolized by phase II enzymes into nontoxic glucuronide and sulfate conjugates in the liver (Zhao and Pickering, 2011). However, excessive doses of APAP can induce acute liver failure and hepatocellular necrosis via a highly reactive metabolite produced by CYP2E1 and CYP3A4 (Jaeschke et al., 2012; Michaut et al., 2014). APAP interferes with the formation of mitochondrial respiratory supercomplexes, and leads to decreased ATP generation and increased reactive oxygen species production (Barbier-Torres et al., 2017). In addition to liver injury, APAP induces renal toxicity via protein binding, oxidative stress, γ-glutamyl cycling, or GSH depletion in humans and rodents (Kennon-McGill and McGill, 2017).
Recently, Toyoda et al. (2018) investigated the 13-week subchronic toxicity of APAP in male F344 and Zucker (lean and fatty) rats. They showed that obese Zucker rats were less susceptible to APAP-dependent toxicity in the liver than their lean counterparts. Therefore, in this study, we conducted 13-week subchronic toxicity studies of orally administered APAP using diabetic GK/Jcl rats and conventional Wistar rats, to evaluate the possible effects of T2DM on the susceptibility to toxicity and the NOAELs in both strains.
APAP (lot no. SLBB2780V, 99.8%) was purchased from Sigma-Aldrich (St. Louis, MO, USA). For administration to rats, APAP was added to a basal diet (MF; Oriental Yeast, Tokyo, Japan). Six-week-old male specific pathogen-free GK/Jcl and Wistar rats were purchased from Charles River Japan (Kanagawa, Japan) and used after 1 week of acclimatization. The animals were maintained in the Division of Animal Experiments, Life Science Research Center, Kagawa University, according to the Institutional Regulations for Animal Experiments. The regulations observed included the best considerations of animal welfare, and good animal handling practices, contributing to the replacement, refinement, and reduction of animal testing (3Rs). The animals were housed in polycarbonate cages with soft chip bedding (Sankyo Labo Service, Tokyo, Japan) in a room with a barrier system and controlled light/dark cycles (12 hr), ventilation (air exchange rate 18 times/hr), temperature (23 ± 2°C), and relative humidity (55% ± 5%). The cages and chip bedding were changed twice a week. All animals had free access to the diet and water with or without test chemicals.
Study designAt the beginning of the experiment, the animals were randomly allocated to 6 groups of 10 or 11 rats each based on their body weights measured immediately before starting treatment. Animals were administered 0, 80, 253, 800, 2530, or 8000 ppm APAP via diets for 90 days. We set the lowest-observed-adverse-effect level (LOAEL) reported in a previous study as the intermediate dose (NTP, 1993), and the √10-fold intervals were applied for the descending dose levels to detect 10-fold differences in NOAELs. The diet was changed once per week. General conditions and mortality were monitored daily, and body weights were measured once a week during the experimental period. The amount of supplied and residual diet were weighed weekly to calculate the average daily food consumption and chemical intake throughout the entire treatment period. All rats were fasted overnight at the completion of the treatment, and anesthesia was induced by inhalation of isoflurane. Blood samples were then collected from the abdominal aorta for hematology and serum biochemistry. The experimental design was approved by the Animal Care and Utilization Committee of Kagawa University, Japan, and the animals were cared for in accordance with institutional guidelines as well as the Guidelines for Proper Conduct of Animal Experiments (approval number #162).
Hematology and serum biochemistryThe following hematological parameters were analyzed using a K-4500 automatic hematology analyzer (Sysmex, Hyogo, Japan): white blood cell count (WBC), red blood cell count (RBC), hemoglobin concentration (Hb), hematocrit (Ht), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), and platelet count (Plt). Serum biochemical analysis of the following parameters was performed by SRL (Tokyo, Japan): albumin/globulin ratio (A/G), total bilirubin (T. Bil), glucose (Glu), triglyceride (TG), total cholesterol (T. Chol), phospholipid (P. Lipid), urea nitrogen (BUN), creatinine (Cre), sodium (Na), chlorine (Cl), potassium (K), calcium (Ca), inorganic phosphorus (P), aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), γ-glutamyl transpeptidase (γ-GTP), and HbA1c.
Organ weights and histopathological assessmentAll animals were weighed then sacrificed by exsanguination after collection of blood samples from the abdominal aorta under deep anesthesia. The collected blood samples were processed for hematological and serum biochemical examinations. ETDA-2K was used as an anticoagulant for the blood samples and serum was collected using a centrifugal separator at 300 rpm for 15 min. Complete necropsy after sacrifice was performed for all animals, and the brain, thymus, salivary glands, lungs, heart, spleen, liver, adrenal glands, kidneys, and testes were weighed. These organs were fixed in 10% neutral-buffered formalin, and paraffin-embedded sections were prepared and stained with hematoxylin and eosin for histopathological examination of the following tissue: skin, mammary gland, sternum with marrow, femur with marrow, spleen, thymus, mandibular and mesenteric lymph nodes, salivary glands, heart, aorta, lungs, trachea, tongue, esophagus, stomach, small and large intestines, liver, pancreas, kidneys, urinary bladder, testes, epididymides, seminal vesicles, prostate gland, bulbourethral glands, pituitary gland, thyroid glands, parathyroid glands, adrenal glands, spinal cord with vertebrae, brain, trigeminal nerve, sciatic nerve, Harderian glands, femoral skeletal muscle, and nasal cavity. The testes and eyes were fixed in Bouin’s fixative and Davidson’s solution, respectively. Bone tissues, including the nasal cavity, vertebrae, sternum, and femur, were decalcified with a mixture of 10% formic acid and 10% buffered formalin for up to 2 weeks. Histopathological assessment was first performed on all tissues of the control and highest dose groups, and the liver and kidney of all remaining dose groups. If a chemical treatment-related change appeared at the highest dose, the relevant tissues from the lower dose groups then also underwent examination.
ImmunohistochemistryThe tissue sections were immunostained using the labeled streptavidin–biotin (LSAB) method and the Ventana HX Discovery system (Ventana Medical Systems, Tucson, AZ, USA). For antigen retrieval, sections were heated in RiboCC Buffer (Ventana Medical Systems) at 95°C for 30 min. The sections were incubated with primary antibody, either the rabbit monoclonal anti-rat insulin antibody (C27C9, 1:800 dilution; Cell Signaling Technology, Boston, MA, USA), rabbit polyclonal anti-human glucagon antibody (prediluted; DAKO, Santa Clara, CA, USA), or the rabbit polyclonal anti-rat CYP2E1 antibody (1:1000 dilution; Enzo, Farmingdale, NY, USA). Incubation with biotinylated goat anti-rabbit IgG secondary antibody (Vector Laboratories, Inc., Burlingame, CA, USA) was performed for 30 min. Primary antibody binding sites were stained brown using a diaminobenzidine (DAB)-HRPO hydrogen peroxidase (H2O2) solution (DAB Map Kit, Ventana Medical Systems). We analyzed CYP2E1 expression because a previous report demonstrated APAP-induced acute hepatotoxicity in mice through the inhibition of CYP2E1 expression (Ito et al., 2006).
Statistical analysisThe Dunnett’s test was used for comparison of body and organ weights, hematology, and serum biochemistry tests. The Fisher’s exact probability test was applied for incidences of pathological lesions. P values < 0.05 are considered to be statistically significant. Statistical analysis was performed using GraphPad Prism version 6.0 for Windows (GraphPad Software, San Diego, CA, USA).
No clinically abnormal signs were noted throughout the experimental period, and all animals survived until the scheduled necropsy. The body weight of Wistar rats significantly reduced with 8000 ppm APAP treatment compared to that of control rats from week 1 to 9; however, this difference was not maintained at week 13 (Fig. 1 and Table 1). GK/Jcl rats treated with APAP showed no change in body weight. APAP treatment did not result in a statistically significant change in the daily food intake for either strain, but APAP-treated rats tended to have an increased food intake (Fig. 1 C and D).
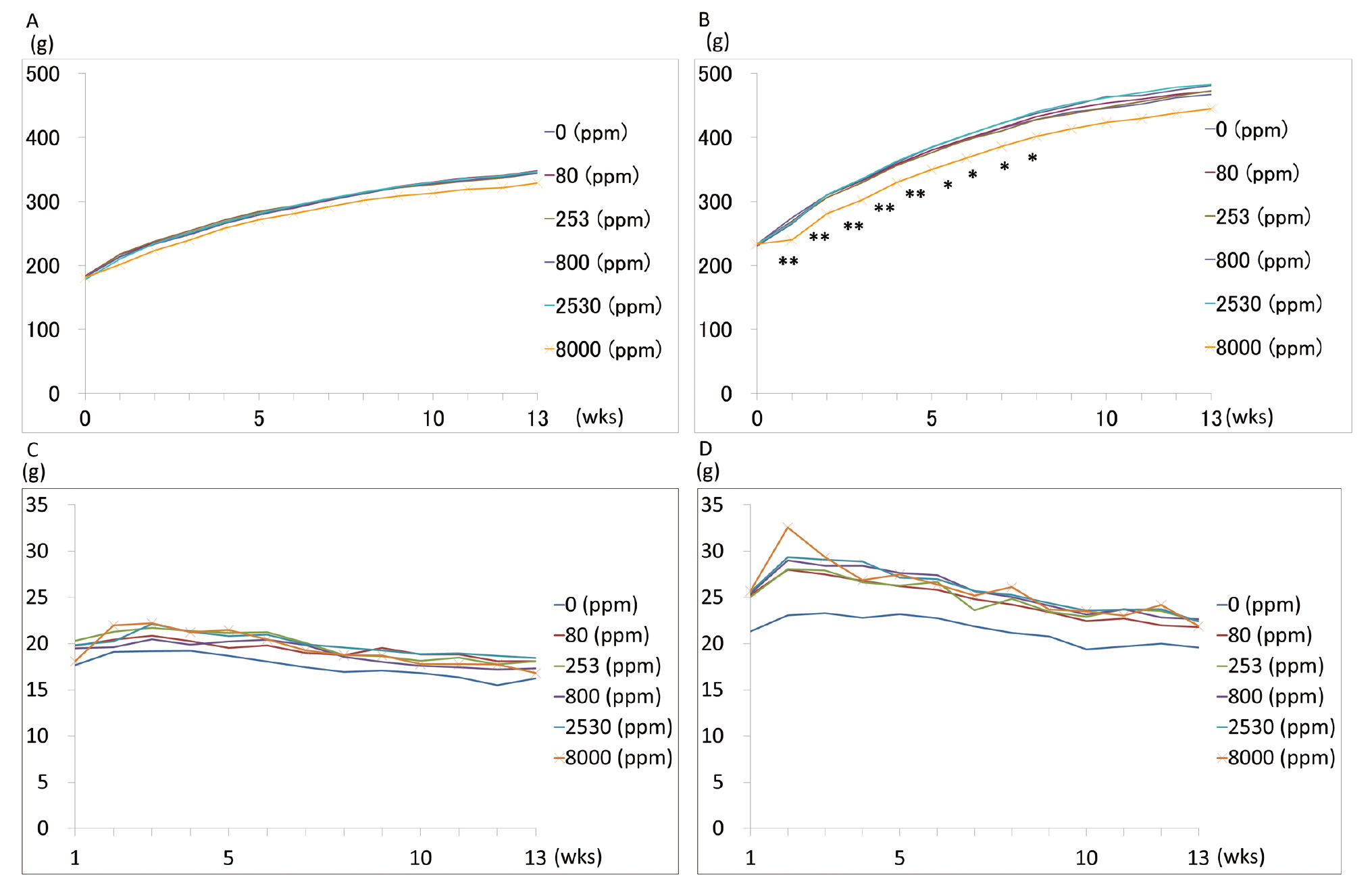
Body weights and food consumption. Body weights of GK (A) and Wistar rats (B). *P < 0.05 vs. 0 ppm. **P < 0.01 vs. 0 ppm. Food consumption of GK (C) and Wistar rats (D).
 Hematology
Hematology
Hematologic analyses are summarized in Table 2. Significant decreases in RBC and increases in MCV were observed in both strains treated with 8000 ppm APAP. A significant increase in MCH was detected in Wistar rats treated with 8000 ppm APAP.
 Serum biochemistry
Serum biochemistry
Data for serum biochemistry are summarized in Table 3. Significant decreases in BUN, Cre, and T. Bil were observed in both strains in response to 8000 ppm APAP treatment as compared to non-treated rats. GK/Jcl rats had significantly higher levels of Ca, and lower levels of TG, Glu, and HbA1c when treated with 8000 ppm APAP when compared with non-treated rats. T. Bil also decreased in GK/Jcl rats treated with 2530 ppm, but not in Wistar rats.
 Organ weights
Organ weights
Absolute and relative organ weights are summarized in Table 4. Significant decreases in the absolute weights of the salivary glands and testes were noted in both strains treated with 8000 ppm APAP. Significant decreases in the relative weight of the salivary glands and increases in the relative weights of the liver and kidneys were noted in both strains treated with 8000 ppm APAP. The relative weight of the testes of GK/Jcl rats also significantly decreased in the 8000 ppm APAP group. In addition, the relative weight of the salivary glands also decreased with 2530 ppm APAP treatment.
 Histopathological findings
Histopathological findings
Histopathological findings are summarized in Table 5. All GK/Jcl rats had irregularly shaped islets of Langerhans (Fig. 2A), whereas all Wistar rats had compact and round islets in the pancreas (Fig. 2D). In GK/Jcl rats, insulin-positive β-cells decreased (Fig. 2B), while glucagon-positive α-cells were not altered (Fig. 2C) relative to those of Wistar rats (Fig. 2E and F, respectively). No differences were observed between the islets of GK/Jcl rats with or without APAP treatment.
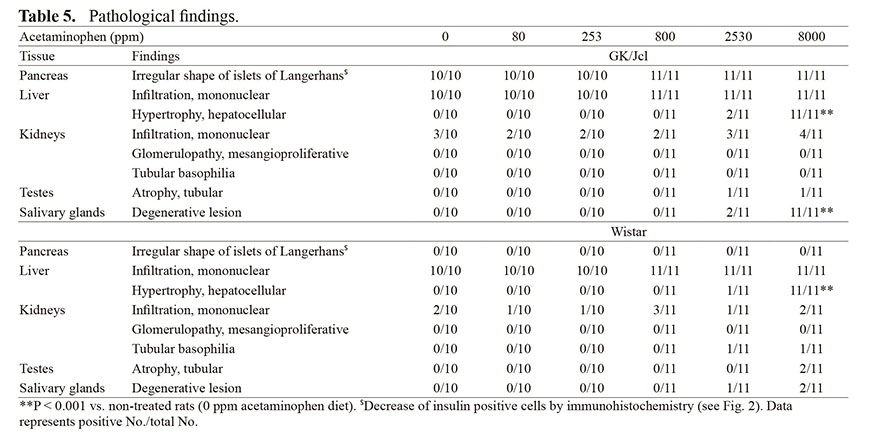
Islet of the pancreas. (A, B, and C) GK/Jcl rat (20 weeks, APAP 8000ppm); (D, E and F) Wistar rat (20 weeks, APAP 8000 ppm); (A and D) H&E; (B and E) insulin; (C and F) glucagon. GK/Jcl rats showed irregular shape of islets with decrease of insulin-positive cells while Wistar rats showed compact shape of islets.
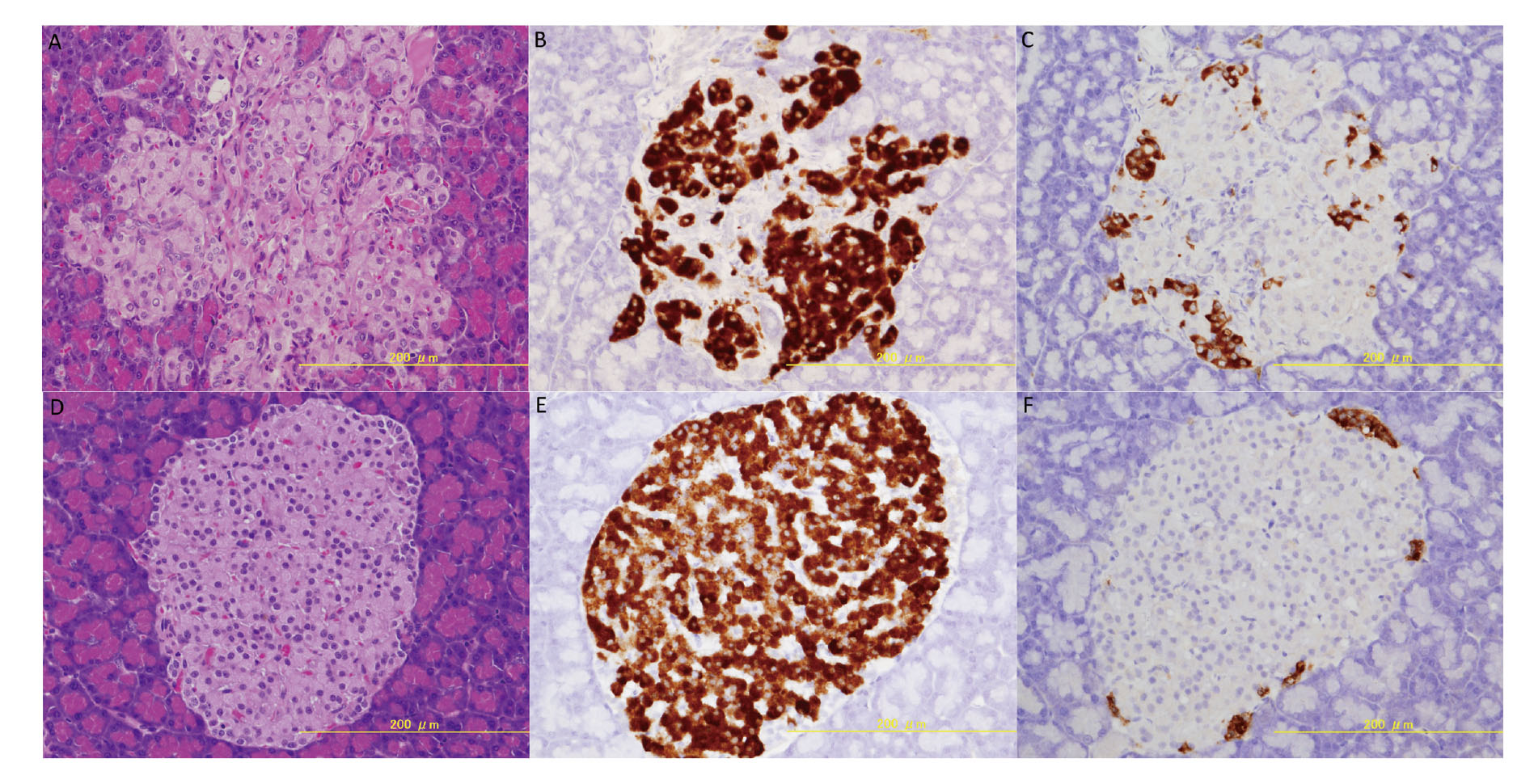
Hepatocyte hypertrophy was observed in both strains of all rats treated with 8000 ppm APAP (Fig. 3B and D, GK/Jcl and Wistar rats, respectively) and in two GK/Jcl rats and one Wistar rat treated with 2530 ppm APAP; however, hepatocyte hypertrophy was not observed in either strain treated with 800 ppm or lower doses (Fig. 3A and C, GK/Jcl and Wistar rats, respectively). The incidence of hepatocellular hypertrophy in both strains treated with 8000 ppm APAP significantly increased relative to non-treated rats (Table 5, P < 0.01). CYP2E1 expression in the liver. (A) 0 ppm and (B) 8000 ppm in GK/Jcl rats. (C) 0 ppm and (D) 8000 ppm in Wistar rats. Both rats treated with 8000 ppm APAP showed increased expression of CYP2E1 in the centrilobular area compared with the perilobular area, but non-treated rats did not show this (Fig. 4, arrows indicate centrilobular area), suggesting that preexisting T2DM and hepatocyte hypertrophy did not affect CYP2E1 expression. Mononuclear cell infiltration was observed in the liver of all animals to the same extent.

Hepatocellular hypertrophy of the liver. (A) 0 ppm and (B) 8000 ppm in GK/Jcl rats. (C) 0 ppm and (D) 8000 ppm in Wistar rats. Both rats treated with 8000 ppm APAP showed enlarged hepatocytes (hypertrophy of hepatocytes), but non-treated rats did not show it.
CYP2E1 expression in the liver. (A) 0 ppm and (B) 8000 ppm in GK/Jcl rats. (C) 0 ppm and (D) 8000 ppm in Wistar rats. Both rats treated with 8000 ppm APAP showed increased expression of CYP2E1 in the centrilobular area compared with the perilobular area, but non-treated rats did not show this. Arrows indicate centrilobular area.
Degenerative lesions were observed in the salivary glands of all GK/Jcl rats treated with 8000 ppm APAP (Fig. 5B), two GK/Jcl rats treated with 2530 ppm APAP, and one and two Wistar rats treated with 80000 and 2530 ppm APAP (Fig. 5D); however, degenerative lesions were not observed in the salivary glands of non-treated rats (Fig. 5A and C, GK/Jcl and Wistar rat, respectively). The incidence of degenerative lesions in salivary glands significantly increased in GK/Jcl rats treated with 8000 ppm APAP (Table 5, P < 0.01), whereas statistical significance was not obtained in Wistar rats.
Degenerative changes in the histology of the salivary glands. (A) 0 ppm and (B) 8000 ppm in GK rats. (C) 0 ppm and (D) 8000 ppm in Wistar rats. Both rats treated with 8000 ppm APAP had pale-colored cytoplasms in acinar and duct cells.
Atrophy of the testes (decreased number of gland cells and sperm) was observed in both strains treated with APAP (N=1 in 2530 or 8000 ppm APAP-treated GK/Jcl rats; N=2 in 8000 ppm APAP-treated Wistar rats). Several other lesions were sporadically detected in APAP-treated rats such as infiltration of mononuclear cells and tubular basophilia of the kidney, while no significant treatment-dependent alterations in their incidences were observed.
This 13-week subchronic toxicity study demonstrated that dietary administration of 8000 ppm APAP resulted in various changes in both GK/Jcl and Wistar rats, including (1) hepatocellular hyperplasia with increased relative liver weight and decreased total bilirubin, (2) increased incidence of degenerative lesions in the salivary glands with decreased relative salivary gland weight, (3) decreased RBC counts and increased MCV, and (4) decreased serum BUN and Cre levels and increased relative kidney weight. Furthermore, 8000 ppm APAP induced a decrease in HbA1c and Glu in GK/Jcl rats, both of which were higher in GK/Jcl rats relative to Wistar rats. In addition, higher levels of Na, Ca, and T-Chol, and a decrease in the weight of the testes were observed in GK/Jcl rats treated with 8000 ppm APAP relative to non-treated GK/Jcl rats; however, these differences were not observed in Wistar rats. APAP 2530 ppm treatment in both strains also induced hypertrophy of the hepatocyte and degenerative changes in histology of the salivary glands, even at low incidence. The NOAEL for APAP was estimated to be 800 ppm for both GK/Jcl and Wistar rats.
GK/Jcl rats represent a unique non-obese and lean model with a manifestation of T2DM that broadly mimics the development of human T2DM. They show fasting hyperglycemia, impaired insulin secretion, insulin resistance, disturbances in the lipid profile, altered heart and body weight, and a variety of late complications, including cardiomyopathy, nephropathy, and neuropathy. GK/Jcl rats had higher values of Glu, HbA1c, low density lipoprotein, high density lipoprotein, and T. Chol, and a lower value of TG than Wistar rats. PMID 23573310 Three genetic loci are responsible for coding and transferring the diabetic pathology to the fetus, and these include genes that are responsible for a reduction in β-cell mass and reduced insulin secretion (Akash et al., 2013). Mutations in the promoter of the adenylyl cyclase (AC)-III gene, overexpression of AC-III mRNA, and enhanced cAMP generation in the islets of GK/Jcl rats have been reported (Abdel-Halim et al., 1998).
Toyoda et al. (2018) showed that hepatocellular hypertrophy was observed in both F344 and lean Zucker rats treated with 8000 ppm APAP, but not in fatty Zucker rats. They concluded that fatty Zucker rats showed a higher tolerance for APAP compared to control rats. Ito et al. (2006) demonstrated that a high-fat and high-carbohydrate diet attenuated APAP-induced acute hepatotoxicity in male C57BL/6 mice through the inhibition of CYP2E1 expression. APAP-induced acute liver injury was mediated by CYP2E1 and the reduction of the liver glutathione content in rats (Tsuchiya et al., 2018). This may explain the higher hepatotoxicity of APAP-treated GK/Jcl and Wistar rats we observed, as CYP2E1 expression was increased in the hepatocytes of APAP-treated GK/Jcl and Wistar rats.
It has been reported that an APAP overdose in glucose-6-phosphate dehydrogenase-deficient individuals causes hemolytic anemia (Rickner et al., 2017). Glucose-6-phosphate dehydrogenase is a critical enzyme in the redox metabolism of RBCs, which enables RBCs to counterbalance the oxidative stress triggered by several drugs, such as APAP. F344 and Zucker (lean and obese) rats treated with 8000 ppm APAP for 13 weeks also had decreased levels of RBCs and increased MCV and MCH (Toyoda et al., 2018), as found in the present study. Anemia may be one of toxic effects of APAP.
Treatment of 8000 ppm APAP improved HbA1c and blood glucose levels in GK/Jcl rats, whereas Wistar rats showed no changes in HbA1c and blood glucose levels. APAP did not induce morphological changes or the number of insulin positive cells in the islets of GK/Jcl rats. Amiri et al. (2015) reported that an acetylsalicylic acid, a nonsteroidal anti-inflammatory drug, improved glucose tolerance and pancreatic β-cell function in GK/Jcl rats. Acetylsalicylic acid reduced the pro-inflammatory prostaglandin, PGE2, which was higher in GK/Jcl rats. APAP and acetylsalicylic acid suppress the production of PGE2 via inhibition of COX activity (Gunaydin and Bilge, 2018). PGE2 may contribute to diabetes via EP3, a PGE2receptor, by inhibiting insulin secretion leading to impaired glucose tolerance and insulin sensitivity (Nasrallah et al., 2016). Taken together, the observed improvement in HbA1c and Glu levels may be caused by the PGE2 inhibitory function of APAP. It has also reported that inhibition of PGE2 improves renal function (Nasrallah et al., 2016); therefore, decreased BUN and Cr in this study may be related to APAP treatment.
Orally administered APAP was secreted into saliva via P-glycoprotein in Wistar rats (Schaiquevich et al., 2004). It may be related to the presence of degenerative lesions in the salivary glands, observed in both strains treated with 8000 ppm APAP. The pathological significance of salivary gland change is unclear, but clinical trials show an increase in salivary secretion in APAP-treated patients as a minor side effect (Miki et al., 1996).
The dose of 800 ppm was set as the intermediate dose since it was the LOAEL with a significant increase in relative liver weights in the 13-week toxicity study using F344 rats (NTP, 1993). The study reported that liver lesions, including hepatocellular hypertrophy and chronic-active inflammation, were found with a high frequency in rats treated with 12,500 ppm APAP, whereas were no hepatic lesions in lower dose (800-6200 ppm) groups. In Zucker rats, a single case of hepatocellular hypertrophy was observed in the 8000 ppm APAP group (Toyoda et al., 2018), suggestive of a high APAP. Taken together, liver lesions induced by a high dose of APAP was considered an adverse change.
In the risk assessment of chemicals, the concept of uncertainty factor has been used to convert the NOAEL into the tolerable or acceptable daily intake (Burin and Saunders, 1999). The 100-fold uncertainty factor represents the product of 2 separate 10-fold factors that allow for interspecies differences and human variability (Renwick and Lazarus, 1998). Intraspecies variations and whether the current 10-fold uncertainty factor for intraspecies differences can account for human variability in these factors have been investigated (Dorne, 2010). Based on our results, the differences in NOAELs for APAP between GK/Jcl and Wistar rats were shown to be within the 10-fold range, and thus supported the validity of current uncertainty factors for intraspecies variation.
In conclusion, our results revealed that T2DM GK/Jcl and Wistar rats had similar NOAEL values for APAP, suggesting that T2DM may not influence NOAEL of APAP. Further analysis might be needed to determine the relationship between T2DM and the susceptibility of certain types of chemicals.
The authors thank Mrs. Sanae Kushida for preparing the manuscript. This study was supported by a grant from the Food Safety Commission of Japan (no. 1204).
Conflict of interestThe authors declare that there is no conflict of interest.